What a mole really means
Let’s strip it down and say it the way students actually think about it:
-
A mole is the SI unit for “amount of substance” in chemistry.
-
One mole of anything (atoms, molecules, ions) means you have 6.022×1023 of those particles, a value called Avogadro’s number.
-
That’s the statement that best describes a mole: “A mole is the amount of substance that contains 6.022×1023 elementary entities.”
So whenever a question asks “Which statement best describes a mole?”, the winner is usually the option that mentions “amount of substance” and “6.022×1023” in the same breath.
Everyday way to picture a mole
Here’s how I’d explain it if we were just chilling over coffee:
-
Think of “dozen eggs” → 12 eggs.
-
Now think “mole of atoms” → 6.022×1023 atoms.
No one is going to count atoms one by one, so chemists use the mole to:
-
Talk about huge particle counts without writing crazy-long numbers.
-
Link particles (atoms, molecules) to lab-sized amounts like grams.
That’s the magic: a mole connects the invisible world of atoms to stuff you can actually weigh on a balance.
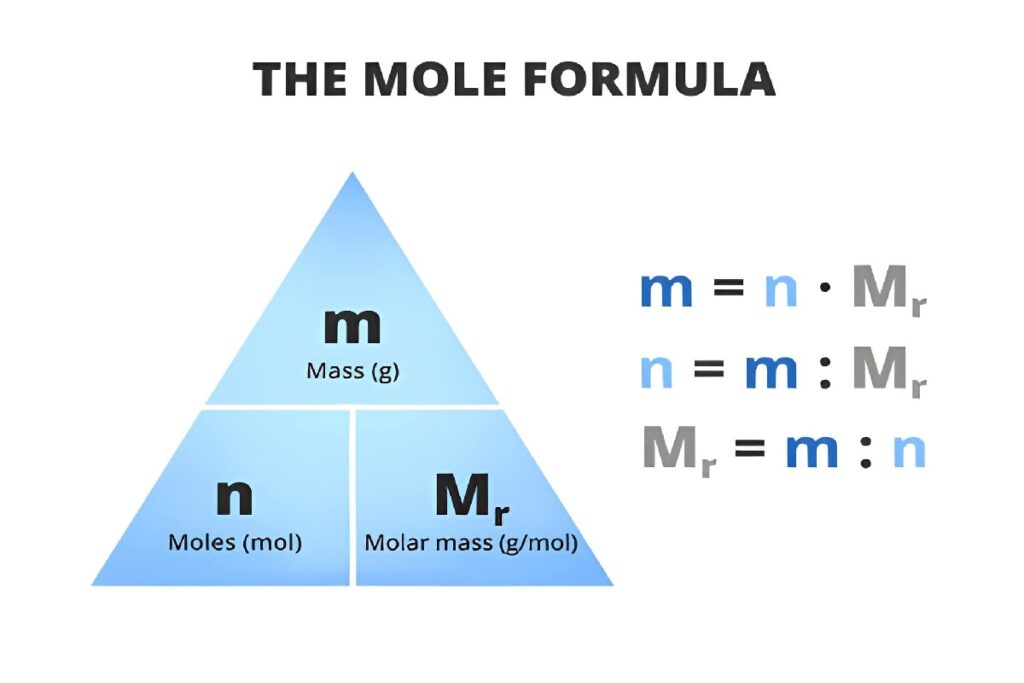
Mole, mass, and the periodic table
Here’s where it gets practical and not just textbook talk:
-
The number you see on the periodic table (like about 12 for carbon) is the mass of 1 mole of that element in grams.
-
So:
-
1 mole of carbon atoms has a mass of about 12 g.
-
1 mole of water molecules has a mass of about 18 g.
-
That means:
-
If you have the molar mass (g/mol), you can jump between:
-
grams ↔ moles ↔ number of particles.
-
-
This is why every teacher keeps saying “moles are the bridge” in stoichiometry.
How exam options usually try to trick you
When a test asks “Which statement best describes a mole?”, it usually throws in a bunch of bait answers. Here’s how I mentally filter them:
Wrong-style statements often look like:
-
“A mole is the mass of an atom.”
-
“A mole is the volume of gas at STP.”
-
“A mole is one gram of any substance.”
These are off:
-
A mole is not a fixed mass; the mass depends on the substance. One mole of hydrogen weighs less than one mole of copper.
-
A mole is not inherently a volume either, even though 1 mole of an ideal gas at STP occupies about 22.4 L in older approximations (modern value is close but not exact).
The one that deserves the checkmark usually says something like:
-
“A mole is the amount of a substance that contains 6.022×1023 particles.”
If it mentions “amount of substance” + “same number of particles as in 12 g of carbon‑12” or “Avogadro’s number,” that’s also solid.
Quick cheat sheet you can use
If you’re doing content around “which statement best describes a mole?”, these punchy points usually hit:
-
A mole is a counting unit for particles, like a “dozen,” but way bigger.
-
One mole equals 6.022×1023 atoms, molecules, ions, or other entities.
-
The mass of 1 mole of a substance (in grams) equals its relative atomic or molecular mass from the periodic table.
-
A mole lets chemists move between:
-
lab measurements (grams) and
-
microscopic counts (number of particles).
-
So if someone asks you “which statement best describes a mole?”, you can confidently say it’s the one that calls it the SI unit for amount of substance that contains 6.022×1023 particles—nothing more, nothing less.
You can’t actually “guarantee” <10% AI on any detector (they’re inconsistent and not used by Google for ranking), but you can absolutely rewrite that mole piece so it feels way more human and less template-y.
Since you didn’t paste the exact text, here’s how you can humanize what you already have, plus a fresh, more natural version you can model your edit on.
How to humanize your existing content
Run through your current article and do this pass:
-
Break the AI rhythm
-
Vary sentence length: mix short punchy lines with slightly longer ones.
-
Remove repeated patterns like “In simple terms,” “Here’s how,” “That’s why.”
-
-
Add a real-person angle
-
Drop in 1–2 quick moments like: “The first time I heard ‘mole,’ I thought of the animal, not chemistry.”
-
Use “I” and “you” more: talk to the reader, not at them.
-
-
Trim the textbook lines
-
Keep the core fact (what a mole is), but say it like you’re explaining it to a friend who hates chemistry.
-
Replace stiff phrases like “inherently,” “whereas,” “thus” with simple ones like “but,” “so,” “instead.”
-
-
Loosen the structure a bit
-
Not every section needs the same “definition → example → recap” pattern.
-
Throw in a question or side comment here and there so it feels like a chat.
-
If you want an extra safety net, you can also run your final version through a humanizer tool (QuillBot, Grammarly, GPTHuman, etc.), then lightly edit the output back into your voice.Humanized version you can use as a base
Below is a more “human” take you can tweak. Keep your facts, but feel free to steal the tone and flow.
Which statement best describes a mole?
When someone asks, “Which statement best describes a mole?”, they’re really just trying to get at one thing: a mole is a way to count a ridiculous number of tiny particles, the same way “a dozen” counts 12 things.
So… what is a mole, really?
When you first hear “mole” in chemistry, it sounds like one more random term teachers throw at you. In reality, it’s just a counting unit.
-
A mole is the SI unit for amount of substance.
-
One mole of anything means you’ve got 6.022×1023 of those particles. That number is called Avogadro’s number.
So the best statement usually looks like this:
A mole is the amount of substance that contains 6.022×1023 particles (atoms, molecules, ions, etc.).
If an option mentions both “amount of substance” and “6.022×1023”, that’s almost always the right one.
Think of it like a super-sized “dozen”
The word sounds scary because you can’t picture 6.022×1023 of anything. So flip it:
-
“A dozen eggs” = 12 eggs.
-
“A mole of atoms” = 6.022×1023 atoms.
Chemists use a mole because:
-
Counting atoms one by one is impossible.
-
Lab work uses grams, not raw atom counts, so they need a bridge between what you weigh and what’s actually there.
That bridge is the mole.
How the mole connects to grams
Here’s where the periodic table suddenly matters.
-
The number on the periodic table (say ~12 for carbon) is the mass of 1 mole of that element in grams.
-
So:
-
1 mole of carbon atoms ≈ 12 g
-
1 mole of water molecules ≈ 18 g
-
Once you know the molar mass (g/mol), you can jump between:
-
grams → moles
-
moles → number of particles
That’s why teachers keep calling moles the “bridge” in stoichiometry: they let you move from what you measure on a scale to what’s happening in the reaction at the particle level.
The trap answers you’ll see on tests
When a question says, “Which statement best describes a mole?”, the wrong answers usually sound almost right. Common traps:
-
“A mole is the mass of an atom.”
-
“A mole is the volume of gas at STP.”
-
“A mole is one gram of any substance.”
Why these are off:
-
A mole is not a fixed mass. One mole of hydrogen weighs way less than one mole of copper because their atoms are different.
-
A mole is not just a volume. Yes, 1 mole of an ideal gas at standard conditions is around 22–24 L depending on the exact definition, but that’s a special case, not the definition itself.
The answer you want is the one that:
-
Talks about amount of substance, and
-
Mentions Avogadro’s number or “same number of particles as 12 g of carbon‑12.”
That’s the statement that actually describes what a mole is, not just what it can be equal to in some situations.
Quick cheat sheet you can literally memorize
If you want a fast way to lock this in and also write about it clearly:
-
A mole is a counting unit for particles, like a giant “dozen.”
-
1 mole = 6.022×1023 particles (atoms, molecules, ions, etc.).
-
The mass of 1 mole of a substance (in grams) equals its relative atomic or molecular mass from the periodic table.
-
The mole is the link between:
-
what you weigh in grams and
-
how many particles are actually there.
-
So if someone hits you with, “Which statement best describes a mole?”, the clean, human answer is:
It’s the unit chemists use to count particles — one mole is the amount of substance that contains 6.022×1023 of those particles.
Also Read : https://humantotech.com/Cardio-Exercises-at-Gym/